- Undergraduate
Bachelor's Degrees
Bachelor of ArtsBachelor of EngineeringDual-Degree ProgramUndergraduate AdmissionsUndergraduate Experience
- Graduate
Graduate Experience
- Research
- Entrepreneurship
- Community
- About
-
Search
All Thayer News

Research Tool Helps Narrow Down Endless Possibilities for New Metal Alloys
May 23, 2023 | by Catha Mayor
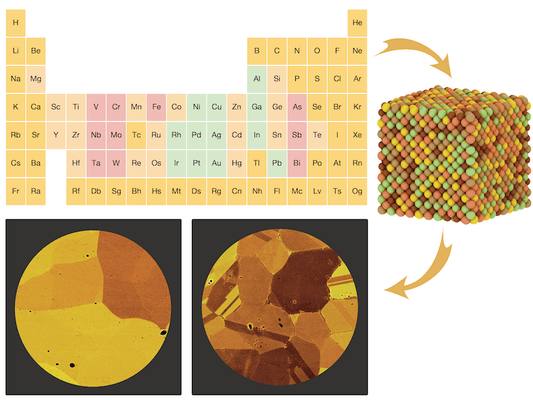
Dartmouth Engineering professor Geoffroy Hautier is part of an international team that developed a physics-based model to help materials scientists decide which metals to try mixing together in the lab. Their method, published last week in Nature Communications, narrows down the hundreds of thousands of possible combinations for new metal alloys, and could significantly speed up the process of finding the next revolutionary material.

SEM image confirming the predicted structure of the synthesized alloy, CoFeMnNiZn. (Image by Wei Chen)
"If you take the forty most common metallic elements and calculate the number of possible five-element combinations—there are more than 650,000 possibilities! Exploring that number purely experimentally is an impossible task," says Professor Hautier. "Instead, we developed a model using thermodynamics and quantum mechanics to predict what combination of elements would mix favorably. Our model found 30,000 new alloys out of 650,000 possibilities. Every new alloy brings new options for technology."
The paper, "A map of single-phase high-entropy alloys," presents these results and discusses not only the type of elements predicted to mix well but also why they would mix. "We tested and validated our model against known alloys, and then used it to make two predictions for alloys that had never been made," says Hautier. "Our team then successfully made these alloys in the lab—an exciting success for modeling, and demonstrates the power of our approach."
Alloys—mixtures of two or more pure metals—usually offer improved properties such as better corrosion resistance, lower costs, higher strength, and better workability. For the most part, alloys don't exist naturally. They must be made. Historically, the creation of new alloys has been a driver of innovation. Modern airplanes, for example, can fly because of alloys that are both light and strong. No alloys, no airplanes.
Traditional alloys consist of a main component with only a small amount of other metals mixed in. For example, steel is mostly iron and carbon with just a small fraction of other metals. During the last decade, interest has grown in going beyond traditional alloys and making what are called high entropy alloys.

"We tested and validated our model against known alloys, and then used it to make two predictions for alloys that had never been made," says Hodgson Family Associate Professor of Engineering Geoffroy Hautier. (Image by Wei Chen)
High entropy alloys are made of at least four different metals in equal or close to equal proportions. For a long time, achieving homogeneity with this many metals was believed to be too difficult and didn't garner much interest. This belief has since been disproven which has opened up a whole new world of possibilities for new materials with intriguing and useful mechanical and chemical properties.
Although hundreds of high entropy alloys have now been made, it's been a guessing game of hit or miss in terms of which metals will mix well and offer the desired properties. "Using our model, my post-doc, Wei Chen, made a website where you can actually type in five elements and get the answer: Will it form or not? And why or why not? So we call it a map because, with so many possibilities, it can help researchers know which direction to go," Hautier says. "At Dartmouth, for example, we plan to use this model to explore alloys that perform well at high temperatures for use in engines to help improve efficiency."
He adds, "The breadth of the study, and the fact that it encompasses forty different elements, makes it unique. By providing all the data within our model, and offering a way to filter by criteria, we hope it will inspire researchers to try mixing elements that nobody had thought of or considered before."
For contacts and other media information visit our Media Resources page.
