- Undergraduate
Bachelor's Degrees
Bachelor of ArtsBachelor of EngineeringDual-Degree ProgramUndergraduate AdmissionsUndergraduate Experience
- Graduate
Graduate Experience
- Research
- Entrepreneurship
- Community
- About
-
Search
All Thayer News

3D Neural Probes Open New Frontiers in Brain Science and Therapy
Aug 21, 2025 | by Catha Mayor
Optimal neural probe design is key for advancing our ability to measure and stimulate neural activity in the brain, and to develop new ways to treat devastating conditions such as blindness and paralysis. A Dartmouth-led study published in Nature Electronics demonstrates an innovative method to achieve three-dimensional (3D) interfacing, and thus solve the long-running "dimensional mismatch" between typical two-dimensional probes and the brain's 3D neural circuits.

Three of the study's lead authors—Yieljae Shin, Yi Qiang Th'23, and Dongyeol Jang—with Professor Hui Fang (r) in Fang's Multifunctional Integrated NeuroElectronics (MINE) Lab at Dartmouth. (Photo by Catha Mayor)
The findings from this research—supported in part by the National Institutes of Health (NIH), including grants from the National Institute of Neurological Disorders and Stroke and the NIH BRAIN Initiative—lay the groundwork for devices that could make it significantly easier to fully decode cognition and impact underlying brain functions.
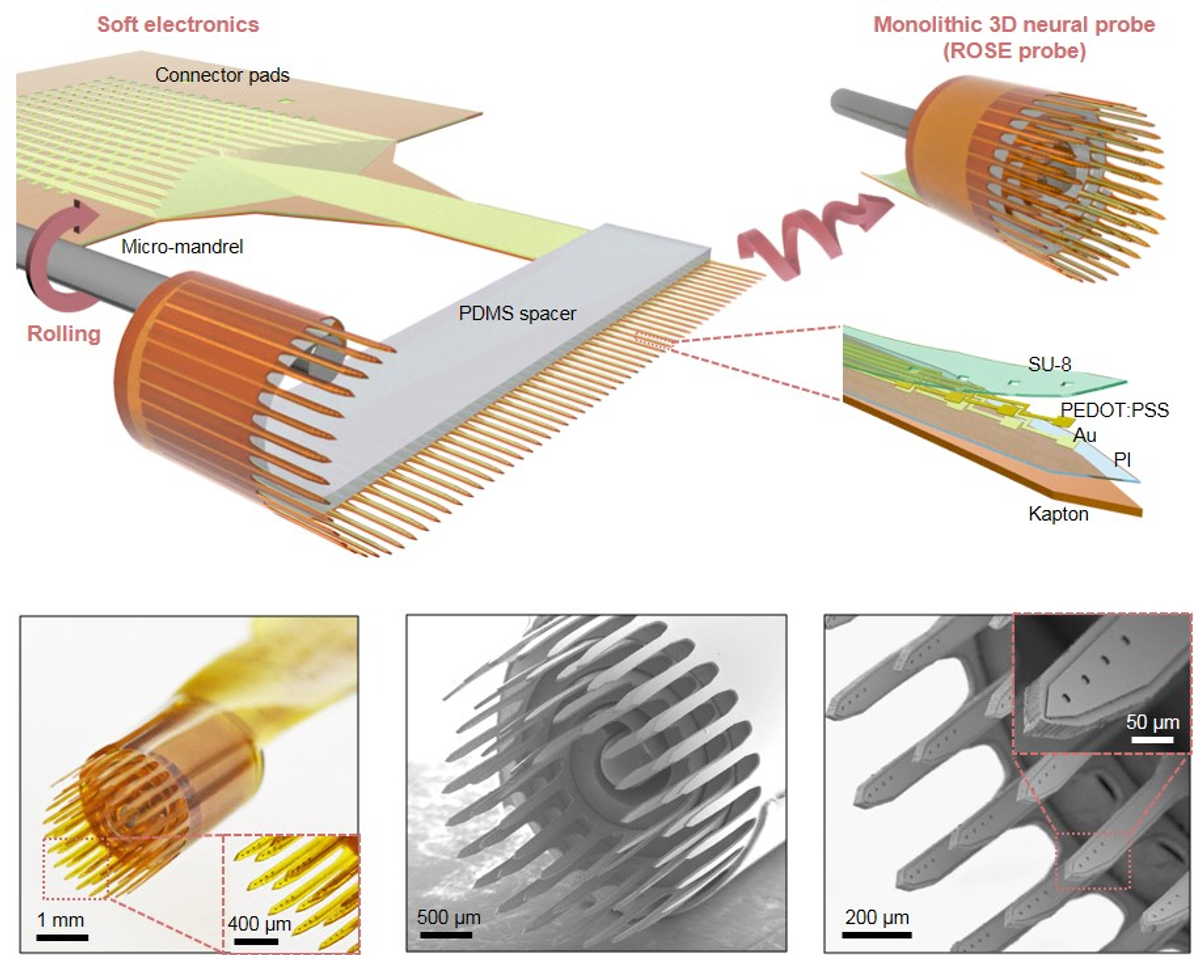
The multidisciplinary team used a unique "rolling-of-soft-electronics" (ROSE) approach to create 3D neural probes that offer both high scalability and unprecedented design flexibility. The study found that ROSE probes not only perform better overall, but also reduce tissue stress and inflammatory reactions compared to traditional 2D silicon probes.
Dartmouth Engineering professor Hui Fang, senior author on the paper, discussed details of the ongoing research:
What inspired you and your team to try and solve the "dimensional mismatch"?
I think it's obvious to everybody, whether they're in the neuro field or not, that 3D interfaces would be much better than 2D. There's no debate about that. So my lab was really inspired by this problem of 3D versus 2D, and then one day it occurred to us that because we're working in this flexible electronics world, why not take advantage of the softness of things? If we roll things up, we can actually realize 3D at the scale of an organ like the brain.
What gives your approach so much design flexibility?
You can think about this like a Swiss roll. Then all these shanks poking out of the Swiss roll will be inserted into the brain, and many different things can be configured—the spacing parameter, the shank pitch, the number of shanks, and the number of electrodes per shank.
Compare that to the gold standard now in our field—the Utah Array—which has only one electrode at each tip. We can have depth profiling electrode arrays along individual shanks, and we can have different designs too. Neurons are tiny and high-density, so from the informational throughput perspective, with our approach you can interface with many more neurons across the shank.

Schematic of a monolithic 3D neural ROSE probe, with the final structure of the probe (top right), and SEM images of the ROSE probe with global and close-up views.
What, exactly, would this be used for?
Devices like this are used either as a neuroscience tool—to enable studies for our colleagues in the Department of Psychological and Brain Sciences, for example—or to serve as a biomedical device. One application for the latter is what's called motor prosthetics. For people with paralysis like tetraplegia, an implanted probe can enable robotic arm movement.
Another emerging application is visual neuroprostheses. So for a blind person whose visual cortex is still intact, you could stimulate the neurons by using patterns related to what a camera can see. You need these devices to provide the right stimulation at the right location for the right neurons in order to produce that type of pattern in the visual, to almost trick the brain so the person will perceive the view as if they can see, which is amazing to me.
I consider this field to be high-risk, high-reward. The full impact is hard to measure, or even imagine, but that's what excites me.
How important is longevity for these devices?
For both fundamental science tools and medical devices, they need to perform for weeks, months, or even years or decades. That's both important and difficult. Implants can fail. Even simple connectors can fail. So we spent a lot of time optimizing those things and carefully documenting all our probe design optimization efforts. We want to share those with the scientific community so people don't have to reinvent the wheel.
There's also a general desire for the probes to be soft, to be mechanically compatible with brain tissue. The entire body is constantly under micro-motion. There's always an agitation between the implant and the tissue. So that's where implant softness matters a lot, because mechanical agitation can cause an inflammatory response and, to the extreme level, can form scar tissue such that it's like an insulating wrap around the probe. That's called encapsulation, and it causes a lot of device failures.
So, besides the major advantages of 3D versus 2D, and the design flexibility, the other main advantage is we use polymer-based electrodes. It's better in terms of mechanical matching, better in terms of alleviating some of the micro-motion-induced incompatibility with the brain. Comparing that to the current gold standard which is made of stiff silicon—I certainly believe the future is polymers in terms of a better material for these brain implantable electronics, especially for biomedical devices.
I'm actually a materials scientist by training. That's my thing. Materials are the foundation of what changes the world.
For contacts and other media information visit our Media Resources page.
