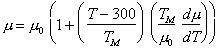THE COVALENT ELEMENTS, Si AND Ge
9.1 General Features of the Mechanical Behaviour of the Diamond Cubic Elements
9.2 Data for Silicon and Germanium
FOUR elements crystallize in the diamond cubic structures: silicon, germanium, α-tin and diamond itself. It can be thought of as an f.c.c. structure with two atoms associated with each f.c.c. atom position. Each atom has four nearest neighbours to which it is linked by four purely covalent bonds. The low co-ordination makes it a very open structure, with a low density. But despite this, the covalent elements are enormously strong, having a flow strength at which exceeds μ/20 at 0 K and which remains high even at 0.5 TM. Their mechanical properties are interesting because they typify the extreme behaviour associated with pure covalent bonding.
Maps and data for silicon and germanium are shown in Figs. 9.1 to 9.4. The parameters used to compute the maps are listed in Table 9.1.
TABLE 9.1 The covalent elements
|
Material |
Silicon |
|
Germanium |
|
|
Crystallographic and thermal data Atomic volume, Ω (m3) Burger's vector, b (m) Melting temperature, TM(K) |
2.00 x 10- 29 3.83 x 10- 10 1687 |
|
2.26 x 10- 29 3.99 x 10- 10 1211 |
|
|
Modulus Shear modulus at 300 K μ0 (MN/m2) Temperature
dependence of modulus, |
6.37 x 104
-0.078 |
(a)
(b) |
5.20 x 104
-0.146 |
(g)
(b) |
|
Lattice diffusion (normal) Pre- exponential, D0υ (m2/s) Activation energy, Qυ (kJ/mole) |
0.9
496 |
(c)
|
7.8 x 10- 4
287 |
(h)
|
|
Boundary diffusion Pre- exponential, δD0b (m3/s) Activation energy, Qb (kJ/mole) |
10- l5 300 |
(d)
|
10- 17 172 |
(d)
|
|
Core diffusion Pre- exponential, acD0c (m4/s) Activation energy, Qc (kJ/mole) |
10- 25 300 |
(d)
|
10- 27 172 |
(d)
|
|
Power- law creep Exponent, n Dorn constant, A |
5.0 2.5 x 106 |
(e)
|
5.0 1.0 x 108 |
|
|
Obstacle-controlled glide 0
K flow stress, Pre-exponential, Activation energy, ∆F/μ0b3 |
—
—
— |
|
—
— — |
|
|
Lattice-resistance-controlled glide 0
K flow stress, Pre-exponential, Activation energy, ∆F/μ0b3 |
0.07
1011
0.2 |
(f) |
0.06
1011 0.2 |
(f) |
|
|
|
|
|
|
|
(a) McSkimin
(1953); Prasad and Wooster (1955); (b) McSkimin (1959); McSkimin and Andreatch (1964);
(c) Masters
and Fairfield (1966); (d) Estimated using Qb=0.6 Qυ and δD0b=2bD0υ; δDb= δD0b exp –(Qb/RT); acDc=acD0c exp - (Qc/RT).
|
(e) Adjusted
to fit Myschlyaev et al. (1969). The constant A refers
to tensile creep; in computing the maps (f) Adjusted to fit the hardness data of Trefilov and Mil'man (1964). (g) Fine (1953); McSkimin and Andreatch (1963). (h) Letaw et al. (1956). (i) Estimated
by using the same creep exponent as silicon and
|
|||
9.1 GENERAL FEATURES OF THE MECHANICAL BEHAVIOUR OF THE DIAMOND CUBIC ELEMENTS |
The mechanical behaviour of Si and Ge is
dominated by their localized, strongly directional, covalent bonding. It
creates an exceptionally large lattice resistance for slip on all slip systems,
including the primary system ![]() (Alexander and Haasen, 1968). The
result is that the normalized flow strength at below half the melting point is
larger than that for any other class of solid (except perhaps ice). It is so
large that, in most modes of loading, covalent elements and compounds fracture
before they flow. The hardness test allows the plastic behaviour to be studied
in a simple way down to 0.2 TM, when fracture becomes a problem here also.
(Alexander and Haasen, 1968). The
result is that the normalized flow strength at below half the melting point is
larger than that for any other class of solid (except perhaps ice). It is so
large that, in most modes of loading, covalent elements and compounds fracture
before they flow. The hardness test allows the plastic behaviour to be studied
in a simple way down to 0.2 TM, when fracture becomes a problem here also.
The localized nature of the bonding has other consequences: the energy of formation and motion of point defects is large, so that diffusion (at a given homologous temperature) is slower than in other classes of solid. At high temperature ( > 0.6 TM) Si and Ge show power- law creep, often complicated by an inverse transient (in creep tests) or a yield drop (in constant strain- rate tests) during which the dislocation density increases towards a steady state. This steady state is rarely reached in the small strains of most published experiments, although Myshlyaev et al. (1969) [1] report steady behaviour in silicon. We have assumed that steady-state creep can be described by the rate-equation used in earlier sections for metals (eqn. (2.21)) and that, at sufficiently low stresses, diffusional flow (as described by eqn. (2.29)) will appear . Both creep mechanisms are diffusion-controlled, and are slow because of the slow rates of diffusion. Thus, the covalent elements and the covalently bonded 3-5 and 2-6 compounds, as a class, are stronger (in terms of the normalized variable σs/μ) at all homologous temperatures, than any other class of solid; the ultimate example, of course, is diamond. The maps are a tolerably good fit to the hardness data except below 0.2 TM (when fracture often occurs round the indenter) and to the high- temperature (>0.8 TM) creep data. At intermediate temperatures, the maps match the creep data less well. Lower yield point and transient creep data, some of which are shown on Fig. 9.3, deviate widely from the predicted steady-state behaviour of the maps (as expected) and were ignored in deriving the parameters used in their construction.
The stress axes of the maps shown in this
chapter have been extended upwards by a factor of 10 compared with the others
in this book. This is because, for the reasons already given, the data lie at
higher normalized stresses than those for most other materials; and because it
allows us to show where certain pressure- induced phase transformations
would occur in simple compression (using the pressure ![]() that would appear in a
uniaxial compression test). These phase transformations could become
important in hardness tests, when the hydrostatic pressure at yield is roughly
three times larger for the same equivalent shear stress,
σs.
that would appear in a
uniaxial compression test). These phase transformations could become
important in hardness tests, when the hydrostatic pressure at yield is roughly
three times larger for the same equivalent shear stress,
σs.
There is extensive literature on the mechanical properties of silicon and germanium. The interested reader should refer to Alexander and Haasen (1968) [2] for a comprehensive review.
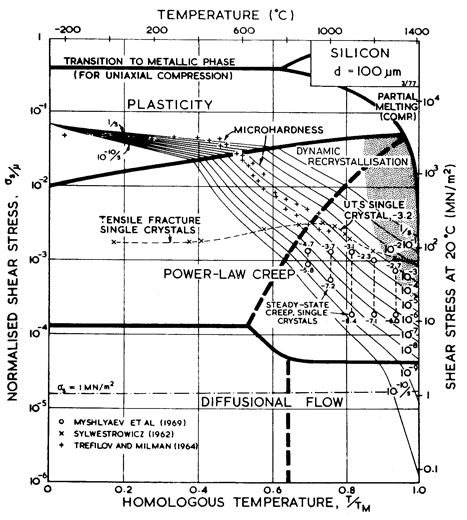
Fig. 9.1. Stress/temperature map for
silicon of grain size 100
µm. Data are
labelled with log10
(![]() ).
).
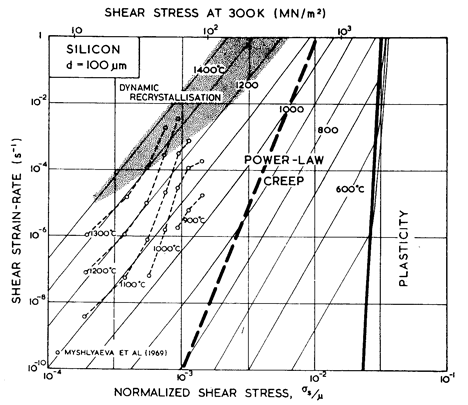
Fig. 9.2. Strain-rate/stress map for silicon of grain size 100 µm. Data are labelled with temperature (°C).
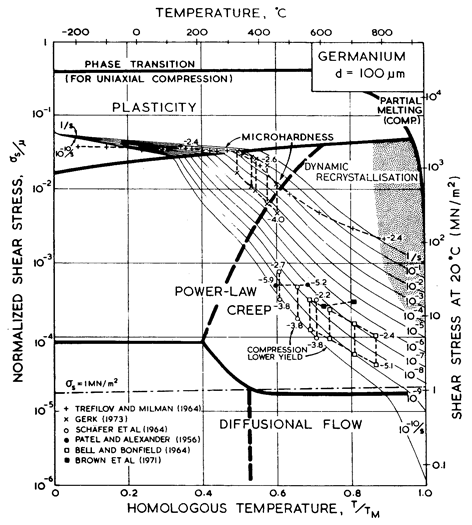
Fig.9.3. Stress/temperature map for germanium of grain
size 100 µm. Data are labelled with
log10(![]() ).
).
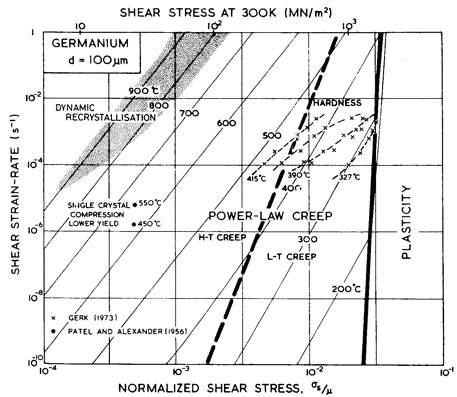
Fig. 9.4. Strain- rate/stress map for germanium of grain size 100 µm. Data are labelled with temperature (°C).
The modulus and its temperature dependence were calculated from single- crystal data using:
![]()
and the data of McSkimin (1953, 1959) [3, 4] and McSkimin and Andreatch (1963, 1964) [5, 6].
Dislocations and slip systems in diamond
cubic crystals have been reviewed by Alexander and Haasen (1968) [2]. Under
normal circumstances, slip occurs on ![]() , giving the five independent systems
necessary for polycrystal plasticity.
, giving the five independent systems
necessary for polycrystal plasticity.
The low-temperature yield strength (T < 0.5 TM) is based on the micro- hardness measurements of Trefilov and Mil'man (1964) [7] corrected according to Marsh (1964) [8]. In both cases the temperature dependence of the hardness is less than can be explained using realistic values of ∆Fp, and the rateequation, using the constants of Table 9.1, does not match the measured hardness below 0.2 TM very well (this may be because fracture occurred at the indenter).
The power- law creep constants for silicon were fitted to the steady- state single- crystal creep data of Myshlyaev et al (1969) [1]. In the range of their measurements they observed an activation energy of between 400 and 520 kJ/mole; that for selfdiffusion is 496 kJ/mole (Masters and Fairfield, 1966) [9] which therefore provides an approximate description of their results. The use of a power- law, too, is an approximation (Fig. 9.2); our values of n = 5 and A = 2.5 x 106 provide the best fit.
We have found no steady- state creep data for germanium. Brown et al. (1971) [10], studying transient creep, found an activation energy of around 340 kJ/mole, which is rather larger than that for lattice diffusion of 287 kJ/mole (Letaw et al., 1956) [11]. Useful information is contained in the high- temperature hardness measurements of Gerk (1975) [12] and Trefilov and Mil'man (1964) [7] which are plotted on Figs. 9.3 and 9.4. Gerk's data suggest a power- law with n = 3; but because the hot- hardness test does not approach steady-state conditions, we have fitted the data to a power- law with the same exponent, n, as that for silicon (namely 5), adjusting the constant A to fit Gerk's data. This choice leads to better agreement with the hardness data of Trefilov and Mil'man (1964) [7] than the alternative choice of n = 3 suggested by Gerk (1975) [12].
1. Myshlyaev, M.M., V.I. Nikitenko, and V.I. Nesterenko, Phys. Stat. Sol., 1969. 36: p. 89.
2. Alexander, H. and P. Haasen, Dislocations and plastic flow in the diamond structure. Solid State Physics, 1968. 22: p. 27.
3. McSkimin, H.J., J. Acoust. Soc. Am., 1959. 31: p. 287.
4. McSkimin, H.J., Measurement of elastic constants at low temperatures by means of ultrasonic waves: data for silicon and germanium single crystals, and for fused silica. Journal of Applied Physics, 1953. 24: p. 988.
5. McSkimin, H.J. and P.J. Andreatch, Elastic moduli of silicon vs hydrostatic pressure at 25oC and -195.8oC. Journal of Applied Physics, 1964. 35: p. 2161-65.
6. McSkimin, H.J. and P.J. Andreatch, Elastic moduli of germanium versus hydrostatic pressure at 25.0 oC and -195.8 oC. Journal of Applied Physics, 1963. 34: p. 651-55.
7. Trefilov, V.I. and Y.V. Mil'man, Soviet Physics Doklady, 1964. 8: p. 1240.
8. Marsh, D.M. Plastic flow in glass. in Proc. R. Soc. 1964.
9. Masters, B.J. and J.M. Fairfield, Silicon self-diffusion. Applied Physics Letters, 1966. 8: p. 280.
10. Brown, D., G. Chaudhari, and P. Feltham, Energy barriers in high-temperature creep of Ge and LiF single crystals. Philosophical Magazine( before 1978), 1971. 24: p. 213-16.
11. Letaw, H., W.M. Portnoy, and L. Slifkin, Self-diffusion in germanium. Phys. Rev., 1956. 102: p. 636.
12. Gerk, A.P., Germanium microhardness and the correlation of indentation creep with dislocation. Philosophical Magazine( before 1978), 1975. 32: p. 355-65.